Hospital Care Studies
The overall objectives of the hospital care studies are to:
- Establish the frequency, disease spectrum and severity, clinical features, management, risk factors, spread and outcomes of SARS-CoV-2 in hospital care in selected European countries;
- Explore hospital care healthcare professionals’ perspectives on the COVID-19 pandemic in selected European countries and identify index subjects for contact tracing and household studies that will deliver the samples and data for the biological and modelling studies;
- Explore opportunities of testing novel diagnostics for SARS-CoV-2 in an IMI2/Wellcome funded hospital care trial already planned to start in the winter season 2020-2021 as part of VALUE-Dx;
- Explore opportunities of testing new therapeutic agents and/or supportive therapy for severe SARS-CoV-2 infection in the ongoing REMAP-CAP trial as part of PREPARE.
Hospital Care Studies Team
The hospital care studies are divided into three different study parts:
MERMAID-ARI
MERMAIDS-ARI 2.0
REMAP-CAP
MERMAIDS-ARI and MERMAIDS-ARI 2.0 are prospective, non-randomised observational studies of acute respiratory infections in selected European countries. The aim of both studies is to establish the prevalence, disease spectrum and severity, clinical features, management, risk factors, spread, and outcomes of SARS-CoV-2 infection in hospital care in European countries. REMAP-CAP is an adaptive platform trial, established as part of PREPARE. Since 2014 an international consortium has built this research-platform designed to be adapted during a pandemic.
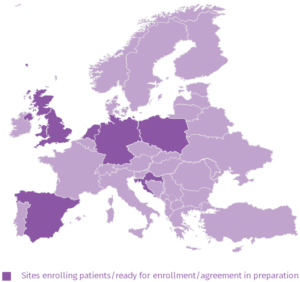 MERMAIDS-ARI
MERMAIDS-ARI
The Multi-centre EuRopean study of MAjor Infectious Disease Syndromes (MERMAIDS) is part of the PREPARE research. This study is looking at acute (recent onset) respiratory (nose, throat and chest) infections, one of the most common infectious diseases across Europe. The study involves a comparison between adults who visit their GP due to respiratory infections and adults who need hospitalisation for similar infections. This will allow us to study why some people develop more severe symptoms. The results of this study can help us to improve the prevention, treatment and care of these infections and hopefully reduce the number of
severe cases.
Respiratory infections such as colds, flu (influenza), and pneumonia affect millions of people around the world every year. Most cases are mild, but some people become very unwell. There is a great deal that we still do not understand about why some people become more unwell than others, including the role of pre-existing health conditions (such as diabetes or heart problems) and the effect of in-born abilities to fight infections. Protocol for the MERMAIDS-ARI study from PREPARE will be re-implemented and will enrol patients presenting to primary care and hospitals with an acute respiratory illness.
The study has a unique opportunity through transcriptome microarray analyses, to identify host and pathogen related determinants of disease severity of ARI, particularly in patients with COVID-19.
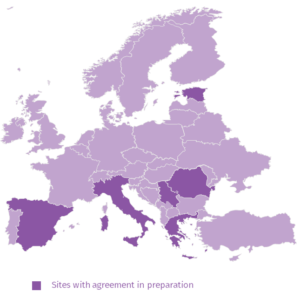 MERMAIDS-ARI 2.0
MERMAIDS-ARI 2.0
This will expand the number of sites and countries participating in an observational study to improve geographical coverage in Europe, enhance accumulation of enrolled cases, allow more and targeted biological sampling, and allow enrolment of children.
REMAP-CAP
REMAP-CAP was designed to be adapted in the event of a pandemic. This is one of the specific goals of the PREPARE funding under which REMAP-CAP was initiated. To this end, a Pandemic Appendix to the Core Protocol (PAtC) has been approved in Europe and globally, already before COVID-19 emerged. This PAtC allows addition of new COVID-19 specific treatment domains and interventions, adaptations of definitions of ICU during a pandemic, adaptation of the primary endpoint (to facilitate quick learning during a pandemic) and adaptation of the statistical model of the trial, deemed necessary during a pandemic.
As planned, new COVID-19 specific treatments are now being investigated. Their incorporation into the platform occurs at an unprecedented speed (and at much lower costs compared to conventional RCTs). The number of potentially lifesaving treatments is considerable, but they all need to be tested in clinical trials. In fact, many of these treatments are being used outside clinical trials, with unknown efficacy and safety.
Careful scientific evaluation of these compounds in a clinical trial is warranted, as continued off-label use may harm patients and will not provide high-quality scientific evidence. Therefore, it is essential that the number of study sites in REMAP-CAP increases throughout Europe. Careful scientific evaluation of these compounds in a clinical trial is warranted, as continued off-label use may harm patients and will not provide high-quality scientific evidence.
Learn more about REMAP-CAP in the introduction video:
- Part 1: a global adaptive platform trial, innovative and easy to handle.
- Part 2: an efficient menu to pick and choose from, including information about interactions.
- Part 3: the REMAP-CAP family, together we can make a difference.
- Part 4: a stadium for clinical research.










